Clinical Care Guidelines
- Introduction Clinical Care Guidelines
- Chapter 1 The Fanconi Anemia DNA Repair Pathway
- Chapter 2 Diagnosis of Fanconi Anemia: Testing and Genetic Counseling
- Chapter 3 Clinical Care of Fanconi Anemia Hematologic Issues
- Chapter 4 Non-HNSCC Solid Tumors in Patients with Fanconi Anemia
- Chapter 5 Head and Neck Cancer in Patients with Fanconi Anemia
- Chapter 6 Oral Health Care for Patients with Fanconi Anemia
- Chapter 7 Gynecologic Care for Female Patients with Fanconi Anemia
- Chapter 8 Dermatologic Issues in Patients with Fanconi Anemia
- Chapter 9 Clinical Care of Fanconi Anemia Gastrointestinal Issues
- Chapter 10 Endocrine Disorders in Patients with Fanconi Anemia
- Chapter 11 Hearing and Ear Issues in Patients with Fanconi Anemia
- Chapter 12 Clinical Care of Hand and Arm Abnormalities in Fanconi Anemia
- Chapter 13 Brief Guide to Clinical Care for Patients with Fanconi Anemia
- Appendix A: Glossary and List of Abbreviations
- List of Contributors
Chapter 11
HEARING AND EAR ISSUES IN PATIENTS WITH FANCONI ANEMIA
Introduction
Hearing and ear anomalies are prevalent in patients with Fanconi anemia (FA). Three of every 20 patients with FA have ear malformations [1] and reported prevalence of hearing loss in patients with FA ranges from 11% to 50% [2, 3]. Hearing loss in patients with FA is typically mild; however, it can impair an individual’s communication abilities and interfere with language development and learning. This chapter will describe common concerns related to ear abnormalities and hearing loss in patients with FA, routine auditory monitoring, amplification tools, and surgical management. The ear and hearing clinical care team for patients with FA should include an otologist and an audiologist and, when needed, a speech-language pathologist. This team should work in close collaboration with other FA specialists and the pri¬mary physician to coordinate care.
HEARING AND EAR ABNORMALITIES IN PATIENTS WITH FANCONI ANEMIA
Researchers at the National Institutes of Health published a study in 2016 on 33 patients with FA who ranged from 3-56 years old to systematically examine and define ear and hearing abnormalities in this patient population [4]. In this study, comprehensive audiologic information available for 31 of the patients showed that hearing loss was detected in 14 (45 %) of the patients: 5 patients had bilateral hearing loss and 9 had unilateral hearing loss. The remaining 17 patients had normal hearing. The majority of hearing loss was classified as mild in degree. Out of the 14 patients with hearing loss, the most common type of hearing loss was conductive, which was found in 9 patients, or 64%. Sensorineural hearing loss (found in 2 patients or 14%) and mixed hearing loss (found in 1 patient or 7%) were less commonly observed.
After detailed microscopic ear examination, structural ear abnormalities were detected in 18 out of the 31 (58%) patients. Narrow ear canals and abnormally shaped pinna were identified in 10 (32%) and 3 (10%) patients, respectively. One patient with FA was born with an absent ear canal, an anomaly known as aural atresia. The most frequent finding was a small tympanic membrane in 18 patients, followed by the short handle of the malleus (manubrium) that was abnormally positioned on the eardrum in 16 patients, and the presence of abnormal bony islands (bony plate) under the eardrum in 12 patients (see Figure 1 as an example). One patient had an underdeveloped auditory nerve and profound sensorineural hearing loss unilaterally. Interestingly, an absent or underdeveloped radius found in 21% of the patients with FA was associated with hearing loss, suggesting a developmental relationship between the radius and structural ear abnormalities [4]. The results from this study indicate the incidence of hearing loss and congenital ear malformation is much higher in patients with FA than previously reported [1, 2, 3]. The findings suggest that abnormal features can be present even if the hearing is normal or only slightly reduced.
In a separate retrospective study of 17 FA patients who underwent a speech in noise (SIN) test, speech reception in noises was subnormal in nine subjects (53%) and abnormal in two subjects (12 %). Two patients with an abnormal SIN test and six patients with a subnormal SIN test had normal audiograms [5].
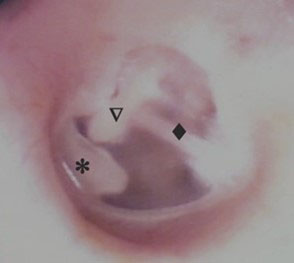
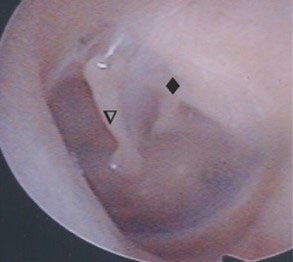
Figure 1. Anatomical differences in eardrums of patients with Fanconi anemia. This figure shows examples of a left eardrum of a healthy individual (left) and a patient with FA (right) with the bony plate (*), manubrium (▽), and chorda tympani nerve (♦) highlighted.
EARLY AND PERIODIC AUDITORY MONITORING FOR PATIENTS WITH FANCONI ANEMIA
Any child diagnosed with Fanconi anemia (FA) should undergo comprehensive assessments of his or her ears and hearing by an otolaryngologist and an audiologist, respectively. Newborn hearing screening tests can miss slight or mild degrees of hearing loss; therefore, all children with FA, including those who pass their newborn hearing screening, should receive follow-up audiologic testing. The earlier hearing loss is identified and treated, the less severe possible permanent effects may be. Research has shown that early identification and treatment (e.g., speech therapy, amplification devices, and educational accommodations and interventions) within the first six months of life can alleviate the long-term adverse effects of hearing loss on learning and language development [6].
Before the age of 3 years, such testing can rule out hearing loss that may affect speech and language development [7]. By the age of 5 or 6 years it is possible to obtain very complete testing across the speech frequencies to rule out a hearing loss that may have subtle effects on communication and learning. A speech in noise (SIN) test should be included as a part of hearing evaluation.
Once hearing loss is identified, the patient’s hearing should be monitored regularly. Babies and toddlers should be seen by an audiologist every 3-4 months, whereas older children should be seen every 6 months until age 6 or 7, after which an annual audiological assessment may be sufficient. If the child’s hearing loss is not stable or if other hearing related issues arise, more frequent monitoring may be recommended. Adults with hearing loss should receive annual audiologic monitoring, or immediate evaluation if they suspect a change in hearing.
It remains unclear whether FA is associated with progressive hearing loss. Therefore, FA patients who have been diagnosed with normal hearing should have their hearing monitored regularly (approximately every 2-3 years). Hearing tests should be performed more frequently in children, because they are unable or unlikely to self-report concerns about difficulties hearing or communicating. Patients with FA are likely to undergo medical and surgical treatments that can potentially affect hearing. Many patients with FA will be treated with medications that are potentially ototoxic. Furthermore, patients with FA are susceptible to recurrent infections due to neutropenia, multiple blood transfusions for severe anemia, and malignancies of the blood and solid tissues; these conditions increase the risk of exposure to ototoxic medications, such as intravenous antibiotics (e.g., aminoglycosides such as gentamicin), iron-chelating agents (e.g., deferoxamine), and chemotherapy agents (e.g., cisplatin). It is important to establish the patient’s baseline hearing level before he or she is treated with ototoxic medications, and to monitor the patient’s hearing closely during treatment. Lastly, the genetic instability associated with FA has been associated with premature aging processes [8]; therefore, patients with FA may be at risk of developing age-related hearing loss at an earlier age than the general population.
CONSEQUENCES OF HEARING LOSS
Children use their hearing to develop speech, language, communication skills, and to facilitate learning, so consequently, hearing loss can interfere with language development and learning. Even slight or mild hearing loss makes it difficult to hear a teacher or peers who are not within close range, especially in environments with a lot of background noise, such as a typical classroom. Left untreated, hearing loss can cause delays in language development and gaps in education. Even if the hearing loss only occurs in one ear and the other ear is normal, a child can have enough trouble hearing in school or in other situations that it impairs his or her social interactions and academic potential [6, 9, 10, 11]. Even minimal hearing loss can negatively impact a child’s social and academic development. A slight to mild degree of hearing loss can make it difficult to understand speech that is not presented at close range, or that is obscured by background noise. Moderate, severe, and profound hearing loss impairs the ability to understand speech under any conditions, and will significantly affect learning and the development of speech and language abilities unless the hearing loss is identified and treated by 6 months of age [12].
Children with hearing impairment often require some form of special education or related services [13]. The United States’ federal Individuals with Disabilities Education Act (IDEA) [14] mandates the development of an Individualized Education Plan (IEP) for any student with a disability who needs special education. Early intervention and academic support teams should work in conjunction with health care providers, such as audiologists and speech pathologists, to identify intervention and academic needs. Section 504 of the U.S. Rehabilitation Act contains provisions for a school-aged child with hearing loss who needs accommodations, such as remote microphone Hearing Assistive Technology (HAT), to access the educational curriculum, but who does not need one-on-one special education teaching or therapy services [15]. This act also contains provisions for workplace accommodations, which should be sought out as needed by employees with hearing loss.
Hearing loss in adults can impair an individual’s communication abilities, especially if the listening situation is not ideal. It can make a person reluctant to participate in conversation and avoid social situations, and can cause fatigue if visual and contextual clues are required to fill gaps between what was said and what was heard.
AMPLIFICATION
If hearing loss is identified in a child or an adult with Fanconi anemia (FA), an audiologist should evaluate the patient’s need for hearing aids and/or hearing assist technologies. There are many different types of devices available. The audiologist will make a recommendation for the appropriate device based on the patient’s lifestyle, type and degree of hearing loss, and the environment in which the device will be used. For example, a school-aged child may need different features on his or her device than an adult in the workforce.
Hearing Aids
Hearing aids are devices worn in or behind the ear that can be beneficial for all types of hearing loss (conductive, sensorineural, or mixed) and almost all degrees of hearing loss. Hearing aids can be used by patients of any age—even babies in their first few months of life. The audiologist programs the hearing aid specifically for a patient’s degree and configuration of hearing loss and can reprogram the device later if the patient’s hearing changes. Hearing aids differ in technology, size, power, and availability of special features.
Hearing Assistive Technology
Remote microphone Hearing Assistive Technology (HAT) helps hearing-impaired individuals function in daily communication situations. They may be used alone or in combination with hearing aids. Hearing assistive technology is typically only used for specific listening situations, such as environments with a lot of background noise (e.g., school classrooms, restaurants, movie theaters, and conferences). The personal remote microphone HAT is a commonly used device that captures sound via a microphone worn by the person speaking. The device then transmits the sound wirelessly to a receiver worn on the ear or attached to a hearing aid. If used in a classroom, for example, the device brings the teacher’s voice directly to the student’s ear at a consistent volume that is above the typical background noise, regardless of the distance between the teacher and student.
Another type of HAT known as a classroom audio distribution system (ADS), or sound-field system, can be a good option for children with hearing loss that is mild or only affects one ear. This system requires the teacher to wear a wireless microphone that transmits sound to speakers that evenly distribute the teacher’s voice to all parts of the classroom. The classroom ADS system can help to ensure that a hearing impaired student can hear what the teacher is saying, even if the teacher is not directly facing the student or is speaking from the other end of the classroom.
Bone Conduction Hearing Devices
A bone conduction hearing device may be useful for patients with conductive hearing loss who cannot use conventional hearing aids due to problems such as a congenitally undeveloped ear canal, or for individuals who are not good candidates for traditional middle ear surgery [16]. For children who fall into this category, such a device can be essential for normal speech and language development. A bone conduction hearing device transmits sound waves directly to the inner ear by vibrating the bone of the skull, which transfers the sound energy to the fluids of the cochlea. A traditional bone conduction hearing aid consists of a bone oscillator or vibrator affixed to a fabric or metal headband that is worn around the head with the oscillator tightly applied to the mastoid bone or cortical bone above the ear. Alternatively, a bone conduction hearing device can be surgically implanted (anchored) into the bone behind the ear in children age 5 years and older.
SURGICAL MANAGEMENT OF HEARING LOSS FOR PATIENTS WITH FANCONI ANEMIA
In the general population, middle ear surgery improves conductive hearing loss in 75% to 90% of carefully selected candidates [17]. It should be noted, however, that sensorineural hearing loss from inner ear or auditory nerve damage cannot be restored by ear surgery.
Below are a few causes of conductive hearing loss that may be surgically corrected in patients with FA:
- Fusion of the malleus to a bony island under the eardrum
- Fixation of the ossicles to the bony walls of the middle ear cavity
- Discontinuity of the ossicles (one of the ossicles is not attached to the others)
- Scarring or bone growth around the stapes
- An absent ear canal
- Fluid in the middle ear
- Perforation of the eardrum
Assessing Candidacy for Surgery
Surgery is not suitable for every patient with conductive hearing loss. Patients with moderate, severe, or profound sensorineural hearing loss are typically not candidates for middle ear surgery. Individuals with serious medical conditions such as heart problems, bleeding tendencies, and a high susceptibility for infection due to bone marrow failure typically are not good candidates for surgery. Candidates for surgery must have normal inner ear function as demonstrated by audiometric thresholds for on bone conduction testing. The otologic surgeon should carefully evaluate the anatomy of the patient’s middle and inner ear using high-resolution thin section CT scanning. This procedure enables the surgeon to determine the possible cause of the conductive hearing loss and gauge the potential success of surgery. In some patients, poor middle ear anatomy or middle ear fluid precludes surgical intervention. It is through both the hearing test and the temporal bone CT scan that a patient’s candidacy for middle ear surgery or canalplasty is determined.
Middle Ear Surgery
Middle ear surgery can be performed in children ages 7 years or older who are capable of cooperating with postoperative care and are beyond the age of frequent childhood ear infections. In patients with an ear deformity known as microtia (in which the external part of the ear, known as the pinna, is underdeveloped or absent), the timing of surgery will depend on the family’s decision regarding reconstructive surgery for the pinna. The options for management of microtia include the following:
- Microtia can be repaired using cartilage from the patient’s ribs. This procedure should be performed prior to middle ear surgery.
- Microtia can be repaired using a synthetic implant, which is often made of high-density polyethylene. This procedure should be performed after middle ear surgery.
- A prosthetic ear can be applied before or after middle ear surgery.
If the middle ear bones are immobile or absent, a surgical procedure called ossicular chain reconstruction can be performed to replace the defective or missing ossicle(s) with a prosthesis. The prostheses are typically made of artificial bone, titanium, or other biocompatible composite materials. Surgery can be done using either local anesthesia and sedation or general anesthesia, and typically takes about one to three hours.
If the ear canal is absent or very narrow, it can be reconstructed in a surgical procedure called canalplasty. During this procedure, the otologist uses an otologic drill to remove bone, thereby opening or widening the ear canal and freeing the ossicles. To restore hearing to the ear, the surgeon constructs a tympanic membrane using a piece of connective tissue. Then the reconstructed eardrum and bone of the ear canal are carefully lined with a very thin skin graft called a split-thickness skin graft. The outer opening of the ear canal, called the meatus, is widened, and the outer edge of the skin graft is delivered through the meatus and sutured to the native skin of the pinna.
Complications associated with ear surgery are uncommon but may include:
- Further hearing loss or no hearing improvement (in less than 10% to 20% of surgeries). Total deafness is extremely uncommon.
- Injury to the facial nerve that runs through the ear, which can cause facial paralysis. This is extremely uncommon. Surgeons should use a device called a facial nerve monitor during ear surgery to minimize this risk.
- Altered taste perception on the side of the tongue, which can last for a couple of months.
- Persistent post-operative dizziness or ringing in the ears, both of which are quite uncommon.
- Restenosis of the ear canal, which requires additional surgery.
Summary
Congenital hearing loss and/or malformations of the eardrum and middle ear are more commonly associated with Fanconi anemia (FA) than reported previously, although the hearing loss is typically mild and conductive. All patients with FA should undergo a comprehensive ear examination and audiologic evaluation by an otolaryngologist and audiologist, respectively. Preferably, these medical providers should be familiar with FA. Hearing problems that are FA-related often can be success¬fully treated with either appropriate amplification and/or surgical correction.
The Fanconi Cancer Foundation recognizes the following author contributions:
H. Jeffrey Kim, MD*
Kalejaiye Adedoyin, MD
Carmen C. Brewer, PhD
Bradley Kesser, MD
Kelly King, PhD
Carter van Waes, MD
Karen L. Wilber, AuD
Christopher Zalewski, PhD
*Section committee chair
- Previous Chapter: Chapter 10
- Next Chapter: Chapter 12
References
- 1. Giampietro et al., The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics, 1993. 91(6): p. 1116-1120.
- 2. Santos et al., Otologic manifestations of Fanconi anemia. Otol Neurotol, 2002. 23(6): p. 873-875.
- 3. Vale et al., Audiologic abnormalities of Fanconi anaemia. Acta Otolaryngol, 2008. 128(9): p. 992-996.
- 4. Kalejaiye et al., Otologic manifestations of Fanconi anemia and other inherited bone marrow failure syndromes. Pediatr Blood Cancer, 2016. 63(12): p. 2139-2145.
- 5. Verheij et al., Hearing loss and speech perception in noise difficulties in Fanconi anemia. Laryngoscope, 2017. 127(10): p. 2358-2361.
- 6. Yoshinaga-Itano et al., Outcomes of children with mild bilateral and unilateral hearing loss. Seminars in Hearing, 2008. 29: p. 196-211.
- 7. American Academy of Pediatrics, J.C.o.I.H., Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics, 2007. 120(4): p. 898-921.
- 8. Suhasini and Brosh, DNA helicases associated with genetic instability, cancer, and aging. Adv Exp Med Biol, 2013. 767: p. 123-144.
- 9. Bess et al., Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear, 1998. 19(5): p. 339-354.
- 10. Bess and Tharpe, Unilateral hearing impairment in children. Pediatrics, 1984. 74(2): p. 206-216.
- 11. Tharpe, Unilateral and mild bilateral hearing loss in children: past and current perspectives. Trends Amplif, 2008. 12(1): p. 7-15.
- 12. Yoshinaga-Itano et al., Language of early- and later-identified children with hearing loss. Pediatrics, 1998. 102(5): p. 1161-1171.
- 13. Americans with Disabilities Act of 1990. 1990, U.S. Statutes at Large. p. 327-378.
- 14. The Individuals with Disabilities Education Act 1997, S.717, 105th Congress. (1997).
- 15. Rehabilitation Act of 1973. 1973, U.S. Statutes at Large. p. 335-94.
- 16. Tjellstrom et al., Bone-anchored hearing aids: current status in adults and children. Otolaryngol Clin North Am, 2001. 34(2): p. 337-364.
- 17. Krueger et al., Preliminary ossiculoplasty results using the Kurz titanium prostheses. Otol Neurotol, 2002. 23(6): p. 836-839.

