Introduction
My subject was born an orphan in 1927. With no known relatives and no connections to a wider community, what followed were 60 years of abandonment. In 1985, one courageous family decided that this isolation had gone on long enough, and that connections to relatives, if there were any, should be found. This family reached out for help from those who were particularly good at understanding this orphan. Investigators chipped away at the mystery and began to uncover clues. The progress seemed painfully slow. Over a 30-year period, bits and pieces of information emerged about a chain of relatives who at first glance seemed distant, then turned out to be close kin. What was once considered an orphan now has an extensive family and has become a celebrity, recognized worldwide. This is the story that I want to tell you.
Fanconi anemia
Some of you may have already figured out that the orphan I am talking about is not really a person, but a disease—Fanconi anemia (FA)—the rare genetic disorder that took the lives of all three daughters of Lynn and Dave Frohnmayer: Katie at 12 in 1991, Kirsten at 24 in 1997, and Amy at 29 in 2016. In 1990, one year after the Fanconi Anemia Research Fund (FARF) was created by the Frohnmayers, National Medal of Science winner David Nathan called this fund the single most effective orphan disease research fund in existence. Fanconi anemia was considered an “orphan” disease, because it was so rare, and so little was known about its causes, its connections to other diseases, or any effective treatment. When Katie Frohnmayer was diagnosed, doctors could only offer palliative care. Some people might have reacted to this shock with paralysis and passivity, but the people in this room know better than most how much intelligence, energy, determination, leadership and sheer grit the Frohnmayers brought to this unwelcome challenge. In the beginning they hoped scientists might uncover “the gene” that causes FA, then do something about it.
When I gave my first talk about FA 14 years ago in 2004, not one but eight genes had been discovered, one of which was BRCA2, a breast cancer gene (see diagram below). That one discovery linked FA to breast cancer, a disease that in 2012 affected 1.7 million women worldwide. Since then, four additional breast cancer genes have also been found to be Fanconi anemia genes. Now we know that BRCA2 is only one of the genes in the Fanconi anemia pathway, all of which, when they function correctly, are essential to the DNA repair process that keeps us alive.
For all of us, the healthy metabolism of our cells requires an elaborate network of interconnected pathways made up of chemical reactions that involve proteins, enzymes, and the synthesis or breakdown of amino acids. When cells fail, it is because something has gone wrong in the DNA replication and repair processes that are governed by these pathways and are essential to life. In people with FA, various genetic mutations lead to malfunctions in this process. If and when we can reverse or prevent these malfunctions, people with FA will have the opportunity to live long, productive lives.
Enter the double helix that Crick and Watson described in 1953, explaining for the first time how cells grow by means of a process in which the two strands of DNA uncoil, duplicate themselves and reassemble in two new double helixes. This process turns out to be very complicated, requiring a multitude of proteins and enzymes to do very specific jobs individually or in concert, at just the right moment and speed, so that everything falls into place. If something goes wrong with this duplication process—which it apparently does an average of 4,000 times a day in every cell in our bodies—a healthy person has an army of chemical soldiers to repair the damage. How do these fix-it soldiers work? They are mobilized not by eight Fanconi anemia genes (as we thought 14 years ago) but by 22 Fanconi anemia genes, each of which plays some role in the DNA repair process for healthy people. It turns out that we all have all 22 of these FA genes, and they are essential to our survival.
In people with Fanconi anemia one or more of these 22 genes has a mutation that makes it defective. Therefore, people with FA have a tougher time than the rest of us repairing DNA damage, whether it is caused internally by normal processes of metabolism or externally, by environmental factors like radiation therapy, chemotherapy, alcohol, tobacco, or the sun. It is important to know that none of us can avoid all DNA damage. No matter how much sun block we wear, no matter how many glasses of wine we don’t sip, or cigarettes we don’t smoke, no matter how many carcinogens we avoid in our air, water, soil, and in the food we eat, our own bodies create DNA damage every single day, just through the normal process of staying alive.
Aldehydes
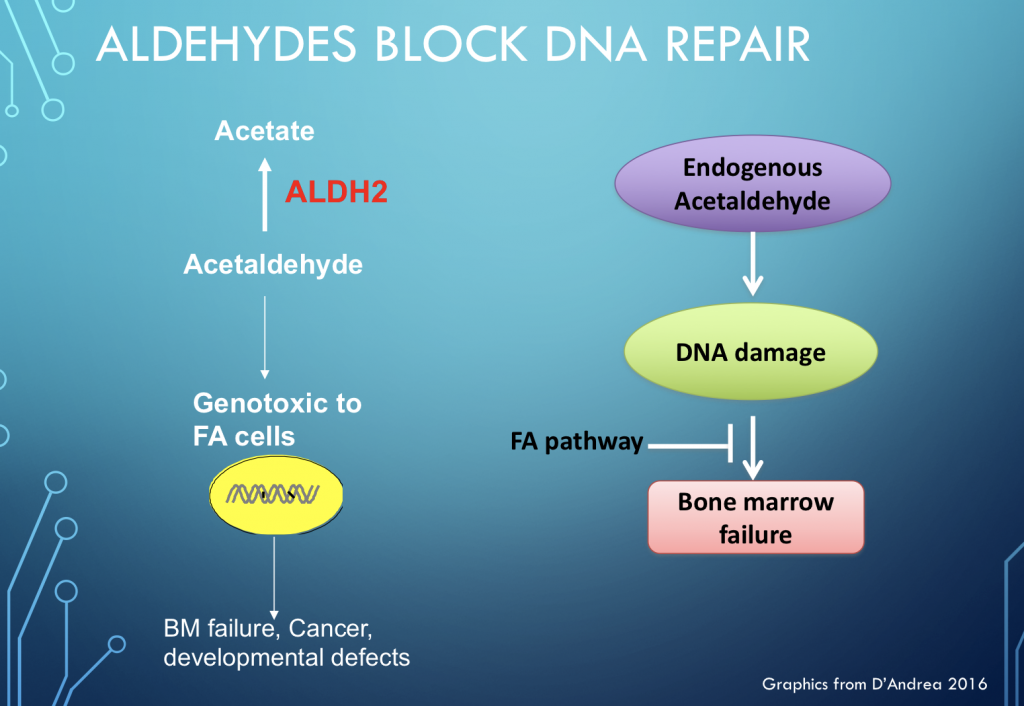
This damage is caused largely by aldehydes, which are small organic molecules with reactive atoms that can bind to DNA and block cell replication. Aldehydes are produced in two ways: by our own bodies, in the course of metabolism (endogenous); or by sources outside our bodies, like alcohol, cigarette smoke, and various chemicals and foods (exogenous).
These reactive aldehydes are a potent source of DNA damage in all people, but people with FA are especially sensitive to this damage.
What this means is that from the moment of conception, when cells are dividing like crazy and life is bursting forth, people with FA begin to fall behind in DNA repair. The damage accumulates at such a rapid rate that babies with FA are sometimes born with missing thumbs, bones, or organs. Though some people with FA show almost no outward signs of the condition, their bodies age prematurely from all this DNA damage. As children they often fall behind on growth charts. As their blood cells struggle to keep up with demand, many kids with FA develop bone marrow failure, or they get leukemia. If a way could be found to restore in people with FA the DNA repair processes that operate in healthy people like you and me to handle the inevitable DNA damage of daily life, Fanconi anemia would become a manageable condition like diabetes or asthma. We would not need to find a cure for FA if the disease itself could be made manageable.
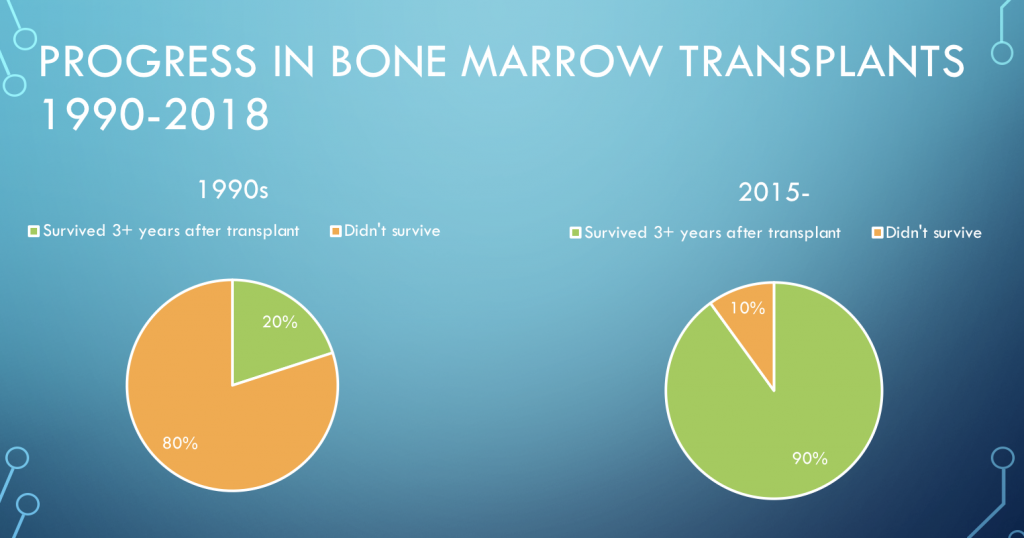
Much of the FA research over the past decades has been devoted not to curing or preventing Fanconi anemia, but to figuring out what it is, and to coping after the fact with the physical problems it creates. In small children, surgeons fashion thumbs out of fingers and straighten out bones that are bending. Doctors prescribe growth hormones that help FA kids grow and that slow down the damage to bone marrow. Still, bone marrow failure remains a huge threat to children with FA. One of the best after-the-fact interventions is a bone marrow transplant that replaces the compromised ability of a person with FA to create healthy blood, with a donor’s healthy ability, contained in donated bone marrow. Because of improvements in bone marrow transplant protocols between 1990, when 20% of FA patients survived the process, and now, when over 90% survive, most children with FA who need a transplant now live into adulthood. This is a remarkable accomplishment. Would that it were a cure!
But for people with FA, the DNA repair problem is not just in the bone marrow, or in the blood. It is in every single cell of the body. A bone marrow transplant can fix the blood, but the rest of the DNA damage grinds on, and the result is all too often cancer. Now that more children with FA are surviving into adulthood, what we are seeing is an epidemic of head and neck cancers in FA adults who have had successful bone marrow transplants. These cancers are particularly awful, because they are painful, disfiguring, and usually lethal. By the time they are detected it is often too late, so FA adults die from head and neck cancer at roughly 500 times the rate in the general population. So far, the best treatments for these head and neck cancers involve detecting them at the earliest possible stage, so that they can be surgically removed before they spread. Dentists, doctors, and surgeons are getting better and better at uncovering them at an early stage, and now robotic surgeries help ferret them out in very hard to reach places, with minimal damage to surrounding tissue. This is fantastic progress. BUT, even if month-by-month and year-by-year diligent patients and doctors catch hundreds of these cancers, the DNA damage continues to pile up, and new cancers appear.
Like bone marrow transplantation, treatment for head and neck cancer is an after-the-fact strategy for dealing with the DNA damage of Fanconi anemia, which is going on in every cell of the body. Doctors may catch and remove some cancers, but new cancers are sure to appear. Successfully treating head and neck cancer, as important as that is, does not cure Fanconi anemia, any more than a successful bone marrow transplant does.
Thank You, Fanconi anemia!
I realize that this all sounds pretty grim, but I am here to tell you that for a number of reasons we should all be grateful to Fanconi anemia and to the research it has inspired. Discovering the link to breast cancer was just the beginning. Now that we know that Fanconi anemia is not just a blood disorder, but rather a DNA repair disorder, and that the 22 Fanconi anemia genes are essential to the DNA repair process in healthy people, these genes turn out to be the very genes that malfunction in various forms of cancer, which is ALSO a DNA repair disorder. Not only are these GENES important, but the metabolic pathways that they are part of, as enzymes are turned on and off in sequence or together, turn out to be critical to the DNA repair process. Uncovering the secrets of FA’s genes and pathways is a step toward uncovering the secrets of all cancers. Right now if you travel anywhere in the world to a scientific meeting that studies blood, radiation, gene therapy, immunotherapy, or various specific cancers, you will hear papers about Fanconi anemia. FA scientists are at the cutting edge of uncovering the secrets of the DNA repair pathways that are needed by healthy cells. In this sense, FA has become a celebrity disease, known to researchers all over the world for the insights it can give them about how cancer destroys lives. What was once an orphan disease is closely connected to a very large family of cancers.
On a therapeutic level, FA research has also found a larger family that includes many people without Fanconi anemia. This is how it works. Because FA patients are extremely sensitive to DNA damage, the toxic regimens of radiation and chemotherapy that are routinely used to treat cancer in the general population, can be lethal for people with FA. By necessity, as doctors have learned how to better prepare children with FA for bone marrow transplants, they have had to find ways to kill off diseased blood and marrow cells using the lowest possible doses of toxic chemicals and the lowest possible levels of radiation. When physicians succeed in doing this for people with FA—thus allowing them to survive bone marrow transplants and reach adulthood—the same less toxic technologies that helped these children can be used to prepare the most vulnerable non-FA cancer patients for surgery or transplantation, avoiding levels of toxicity that might otherwise kill the very sick or the elderly. Thus FA treatments wind up saving the lives of elderly and otherwise vulnerable cancer patients who do not have FA.
Furthermore, because the accumulation of DNA damage in people with FA leads to premature aging, research into the nature of this process has given scientists insights into aging itself, which turns out to be a gradual loss of our ability to repair the damage to our DNA that accumulates day by day over many years. If we could slow that process, what would that mean for the human life span not just of people with FA, but for us all? The discoveries of researchers studying this premature aging process are unlocking secrets about aging that widen the FA family to all of humanity.
Research today
The most cutting edge FA research today is devoted to trying to discover ways not just to correct bone marrow failure, remove head and neck cancer, or repair birth defects—all after-the-fact interventions—but to slow down the DNA damage that is accumulating and to restore to good health the DNA repair process that could prevent these problems from arising in the first place. To that end, a number of promising paths are opening before us.
In a clinical trial for gene therapy going on right now (2018), scientists in Madrid, Spain, are modifying viruses to use them as delivery systems to penetrate the nuclei of cells of people with FA and replace these defective genes with healthy ones. They have done this successfully with four children with FA. As I speak, healthy blood cells are being produced in all four of them, and in two of them, these healthy cells seem to be edging out defective blood cells, which are by definition weak. We are watching these results with excitement and hope, because if this gene therapy turns out to be successful, these children will gain the ability that healthy people have to repair DNA defects in their blood. Preliminary results of this trial look encouraging. Think about it. These children may be able to avoid the whole bone marrow transplant process, which, although much less dangerous than it once was, still requires toxic chemicals to kill off defective bone marrow and blood cells, and every transplant risks Graft v. Host disease, as donor cells attack the tissues in the patient. Bone marrow transplant survivors often live with chronic, sometimes life-threatening problems from this process. If the Madrid gene therapy trials prove as successful as we hope, they could one day eliminate the need for bone marrow transplants in people with FA.
As wonderful as this gene therapy sounds, like bone-marrow transplantation, it affects only the blood, and it does not cure the underlying genetic defect in every cell that is Fanconi anemia.
Immunotherapy
One of the most promising tools on the horizon that could possibly lead to a cure is immunotherapy, which is designed to unlock the human body’s potential to recognize and kill cancers that have been able to put up decoys to block the immune response. Right now there are clinical trials of checkpoint inhibitors that free the immune system to attack cancers, including head and neck cancers. These inhibitors disrupt signals that allow cancer cells to hide from the immune system. By blocking those signals, these inhibitors enable the immune system to attack the cancer cells. A combination therapy of two different checkpoint inhibitors has revolutionized the treatment of metastatic melanoma, with combination therapy achieving a cure rate of approximately 50 percent. Checkpoint inhibitors are also turning out to be effective against some lung, colon, and ovarian cancers. They seem to be most effective against tumors with the greatest numbers of mutations. All this is very promising for people with FA. BUT some people are resistant to immunotherapies, and much more will need to be done to come up with safe and effective treatments.
What can we hope for?
What we are hoping for is a breakthrough that will make it so that we are no longer just reacting ex-post-facto to an inability to repair DNA damage, much of which is an inevitable side-effect of living itself.
Since we can never hope to prevent all DNA damage (which is part of our natural metabolic processes), we are hoping for a breakthrough that will allow us to enable people with FA to cope with that damage as effectively as you and I do. That would constitute a cure for Fanconi anemia!
I can imagine a day in the future when a pregnant woman whose fetus tests positively for one FA genetic defect or another might get a micro injection that repairs that genetic defect in utero before it leads to birth defects and long before bone marrow failure or head and neck cancer appear. Maybe the CRISPR/Cas9 gene editing techniques being studied at UC Berkeley and other research centers will bring that day closer. Maybe some other approach made possible by advances in nanotechnology or viral delivery systems will turn out to be crucial. It is not beyond the realm of credibility to think that such an intervention might one day be possible, sooner rather than later.
You might be wondering, “If we can now test for the 22 genes that can cause FA, why not just have everyone tested, and then people with the same rare defective recessive gene could avoid getting married and risking their one in four chance of having a child with FA?” Here’s the problem. This kind of genetic testing is not yet easily affordable. Furthermore, with 22 FA genes, any one of which might be mutated, it turns out that far more people are thought to be carriers of Fanconi anemia than was once imagined. Is it practical to test everyone? To make matters worse, the DNA repair pathways that the genes are part of are now thought to be as important as the genes themselves. Much remains to be discovered about this dangerous disease and the cancers that share so many of its characteristics.
As the research pushes forward to unlock the secrets of how to prevent Fanconi anemia cancer, we work hard to find more effective treatments for people with FA, whether that means bone marrow transplant protocols, or treatments of head and neck cancer and other cancers. In early 2018 FARF funded its first clinical trial, focused on metformin, a widely available drug that is already FDA-approved for the treatment of type II diabetes and insulin resistance. This drug is an anti-oxidant that has shown documented success in decreasing the incidence of certain types of solid tumors. FARF’s clinical trial of metformin, co-funded by a major grant from the NIH, will assess safety, tolerability, and the biologic effects of this drug in people with FA. In mice studies at OHSU, metformin significantly delayed the onset of cancer, reducing aldehyde toxicity and DNA damage. Will it have a similar effect in people with FA? We sincerely hope so!
Meanwhile, research has shown the benefits of lifestyle changes that can help not just those with FA, but all of us, to reduce exposure to toxic aldehydes that cause DNA damage.
Conclusion
- Avoid pesticides, fertilizers, toxic waste, fossil fuels, sunlight, tobacco, or alcohol, all of which create damage from toxic aldehydes.
- Increase DNA repair resilience through proper rest, healthy eating, exercise, meaningful work, and strong social relationships.
- The Amy Frohnmayer Effect: show gratitude for the miracle of life.
Fanconi anemia was once an orphan disease that no one knew anything about, except that it was rare, it affected the blood and it was fatal. Thanks to 30 years of research tirelessly promoted by the Fanconi Anemia Research Fund, Fanconi anemia is no longer an orphan disease. The DNA repair problem at its center turns out to be the problem that characterizes all cancers. Thus FA is deeply embedded in a huge family of conditions that touches us all. Not only that: the cutting edge discoveries about DNA repair, bone marrow transplant protocols, immunotherapy, gene therapy, and aging, give Fanconi anemia a family so large that it embraces us all. It’s still a fatal disease, but the urgency we feel to find a cure is an urgency not felt in isolation, as it was for the Frohnmayers in 1985. It is an urgency that lies at the heart of our shared mortality.
Sharon Schuman, PhD, University of Chicago, was Secretary of the Board of Directors of the Fanconi Anemia Research Fund. She was Amy Frohnmayer’s violin teacher from age 9 to 15. This presentation was first delivered to the Round Table town-gown organization in Eugene, Oregon, in February, 2018.




